THE ROLE OF CHROMOSOMES IN CHIRONOMID SYSTEMATICS, ECOLOGY AND PHYLOGENY
by WOLFGANG F.WUELKER , Freiburg
A) Introduction
B) Systematic aspects
C) Ecological aspects: formation of ecological niches
D) Parasitological aspects: genetic sex of phenotypic intersexes
E) Zoogeographical aspects: Migration and distribution
F) Phylogenetical aspects
G) Conclusion
Literature Cited
Abbreviations
NOTE: Click on images to see full-size pictures
Chironomid midges are - as other Diptera - outstanding by possessing giant chromosomes. These are up to a quarter of a mm long, which means they are visible under very low magnification. They are found in many cells (mainly in salivary glands, but also present in gut cells, Malpighian tubules, etc.). They are developed in principle by endomitotic replication of chromonemes without cell division, and in consequence built up by bundles of about a thousand chromonemes. Moreover, they remain in interphase nuclei in a status of somatic pairing, i.e. are in an apparent haploid number.
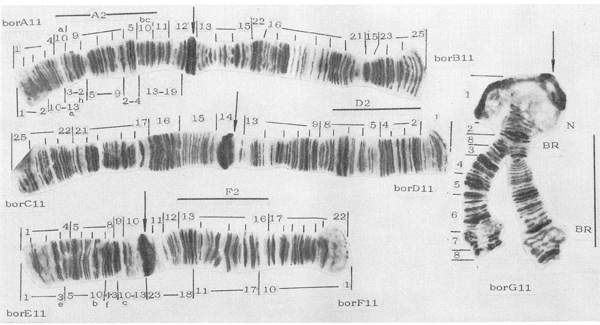
Giant chromosomes have characters (Fig.1):
For a systematic comparison of Chironomid species, it is important whether or not bands of one species can be "homologized" with those of an other species. I show the problem in arm A of different Chironomus species (Fig. 2). With a single band the homologization is nearly impossible: the differences in size and appearance are too small and small deviations can not be excluded. Keyl (1961) called them "structural modifications," which means different appearance of single bands in band groups. He pointed out (1966) that the single band is a "replicon", a unit which can principally replicate alone without influence of the neighboured bands. But groups of bands can be better regarded as equal.
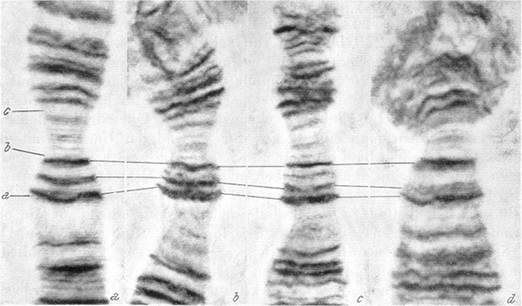 |
Comparing species by chromosome banding patterns has advantages and disadvantages. Positive is the absence of direct modificatory changes (if excluding brutal chromosome damage due to x-rays, radioactive wastes below atomic plants, heavy metals etc.). Negative may be extreme genetic variability in form of inversion polymorphism: inversions produce many new patterns, and selection sorts them out. If on this way a chromosome arm is present in up to 20 variants combined in many heterozygote configurations, recognition of the limits of species may become difficult.
I will now show in this paper some successful uses of chromosomes in different fields of biology.
B) Systematic aspects: finding new species
1) Finding new species
Many new species were identified first by their characteristic chromosome structure. If for instance material of one "species" turned out to contain two karyotypes (C. pilicornis-C. heteropilicornis), this is an indication of presence of two species. If an unusual or rare chromosome arm combination has been detected (C. columbiensis in the new columbiensis-cytocomplex in South America, AG,BF,CD,E or the BAG-chromosome in C. beljaninae), the species in question turned out to be new. Moreover, it may be remembered, that some species mentioned in Keyl (1962) were for a long time known only by 3 characteristical chromosome arms (A,E,F). Description of adults was planned by K. Strenzke, but he died suddenly. The use of chromosome squashes as species types, however, is often practizised, but may still be a matter of discussion.
2) Correcting errors
Nobody of us is perfect, even the great Thienemann. I may be excused if I cite one of his rare errors. He has made the well known pioneer excursion to the Alpes in 1936 and visited a nice place at the foot of Wetterstein mountain, the Schachen-castle of Ludwig II, nearly 1900 m high. In pools nearby he found Chironomus which was sent to Goetghebuer and identified as C. alpestris. I was following the footsteps of Thienemann in 1968-72 with the cytological result that he had combined errously larvae and adults of two species, the larva belonging to C. lacunarius n.sp. and the adult to C. dorsalis (Wülker and Klötzli 1973)
3) Rearranging groups
The decorus-group of Chironomus was first defined by similarity of adults with Chironomus decorus Johannsen (following Townes 1945), but was containing species of different cytocomplexes (maturus-complex: maturus, whitseli, fundatus, wulkeri), pseudothummi-complex C. calligraphus, anonymus, columbiensis-complex C. columbiensis, thummi-complex C. obtusidens, utahensis, acutiventris and 7 other species). Much better was the concept of a cytologically defined, possibly monophyletical decorus (obtusidens-) group confined to the thummi-cytocomplex (Martin 1979, Wülker et al. 1991).
4) Need of integrated treatment
The difficult taxonomic situation in certain species needs integrated investigation of morphological, cytological, biochemical (hemoglobins, enzymes) and recently molecular biological characters.
C) Ecological aspects: formation of ecological niches
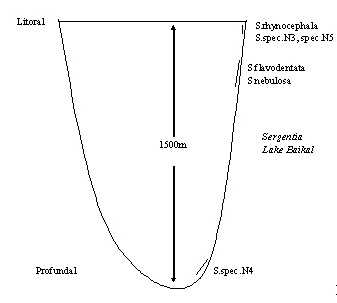 |
Imagine investigations of endemic Sergentia-species in Lake Baikal in Siberia. The genus is radiating in this lake and differentiated at least 12 different species. Some of these species could be described as adults by Prof. Linevich (1981), but this does not allow a judgement about the ecological situation of the larvae. The lake is up to 1500 m deep and collection and rearing of larvae difficult. But cytologic investigations (Proviz et al. 1994) could show that stenobathic littoral species, stenobathic species near the limit sublittoral-profundal, and stenobathic profundal species are present as well as eurobathic elements (Fig. 3). This explained how so many species can coexist in the lake, having remarkable differences in size, presence of eye spots etc.
D) Parasitological aspects: genetic sex of phenotypic intersexes
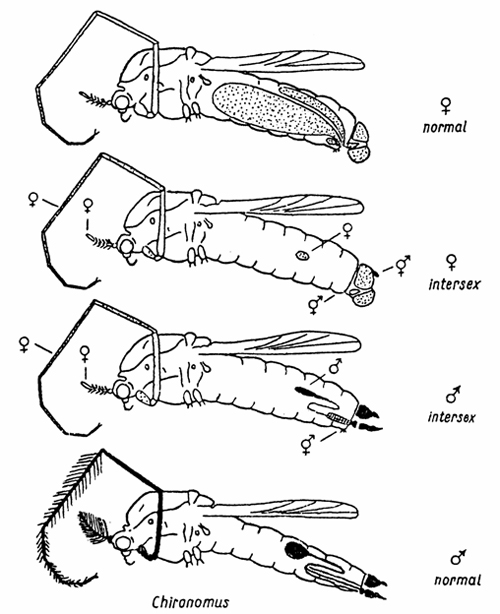 |
Rempel (1940) found in Chironomus "hyperboreus" (later C. rempeli Thienemann) nematode-infested specimens having all a more or less female appearance, only one part had a male hypopygium (Fig. 4). He concluded that all these specimens were genetic females; in some of them the parasite feeds on the gonads, and based on bisexual potency male characters like a hypopygium are formed. I had doubts on this explanation (Wuelker 1961) because specimens with female versus male genitalia occured in Chironomus anthracinus always in a 50:50 percent relation, corresponding to a normal sex ratio. The check of this opinion was possible by a simple trick: We transfered the parasite to a species with sex specific inversions (Camptochironomus), and W. Beermann in Tübingen could show from the chromosomes that indeed specimens with male hypopygia are genetic males, and specimen with female genital appendages genetic females.
E) Zoogeographical aspects: Migration and distribution
The extended cytological investigation of the genus Chironomus in Siberia show exteme inversion polymorphismus of several species (C. plumosus, C. balatonicus, C. acutiventris). Moreover, many species of the plumosus-group are present there, in contrast to the Nearctic, where only plumosus and entis are present (other species like C. vancouveri, C. muratensis and C. balatonicus are noted, but doubtful) and Europe, where C. plumosus and C. balatonicus only seem to be frequent, C. entis, C. muratensis and C. nudiventris seem to be rare and are less inversion polymorphic, as far is known.
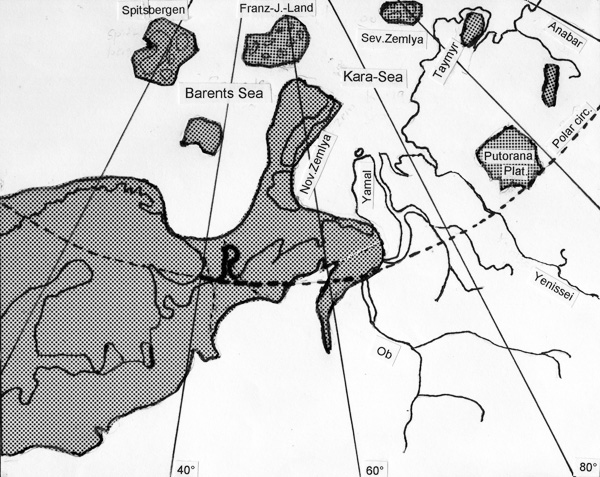 |
Siberia is supposed to be only moderately covered by ice during ice ages (Fig. 5), so may have offered opportunity for radiation in these times. This is in good agreement with the opinion of Guryev et al. 2001 that the origin of the genus Chironomus was apparently South-Eastern Asia and Australia and from there it migrated to Sibera, later on to Europe and the Nearctic.
Comparative investigations of chromosome pattern in the Palaearctic and Nearctic began with Acton (1959) on the two Camptochironomus-species tentans and pallidivittatus. The result was remarkable differences in the respective populations of the two species, but not reaching the level of vicariating species. More recent investigations found differences in species rank between palaearctic and nearctic populations of at least C. tentans Kiknadze et al. 1996, Shobanov et al. 1999).
In C. plumosus (Butler et al. 1999) and C. entis (Kiknadze et al. 2000) the population differences are much less, but higher number of structural types and polymorphismus in Siberia indicates a migration from there to the Nearctic. Also the European populations of C. plumosus are apparently marginal populations of the large distribution area in Siberia.
 |
1) Wagner nets, central and peripheral position of banding patterns, predictions
Morphological investigations in the genus Chironomus, be they with adults, pupae or larvae, brought keys useful for identification, but did not result in ideas about the phylogeny of species.
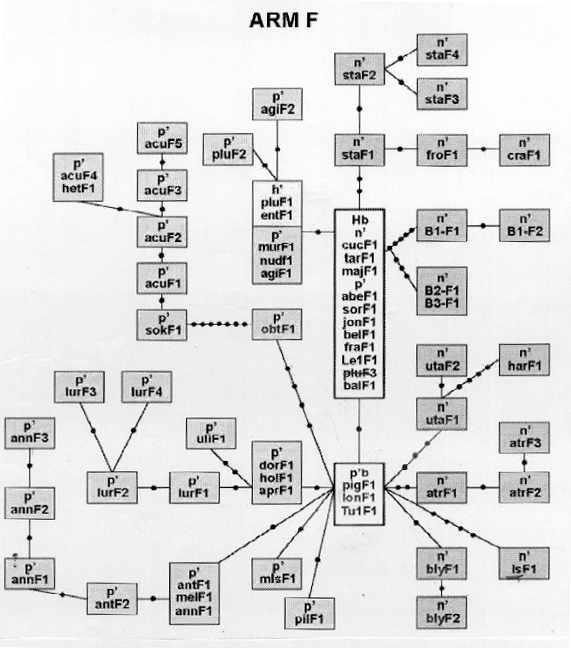
The comparison of chromosome pattern in different Chironomus species resulted first in so-called Wagner-nets, classical examples are Keyl 1962, Martin 1979 or the unpublished net of Kiknadze shown in Fig. 6. They point out the phylogenetic relationships and distances between the species, expressed in inversion steps, also the "central" position of some species is evident. Earlier Keyl (1962) saw the central position of certain species, e.g. C. holomelas in pseudothummi-complex, but warned at the same time, to regard the data about inversion steps as indication of the direction of evolution, because Standard C. piger is choosen arbitrarily. With help of the species positioned at the margin between different cytocomplexes, he tried to characterize the hypothetic species which passed the translocation limit beween thummi- and pseudothummi-cytocomplexes. It may have had the Standard pattern of C. piger in Arm F, the inversion form 12-4 and subsequent 10-2d in arm A and the forms 5-10b, 10b-3f and Standard in Arm E.
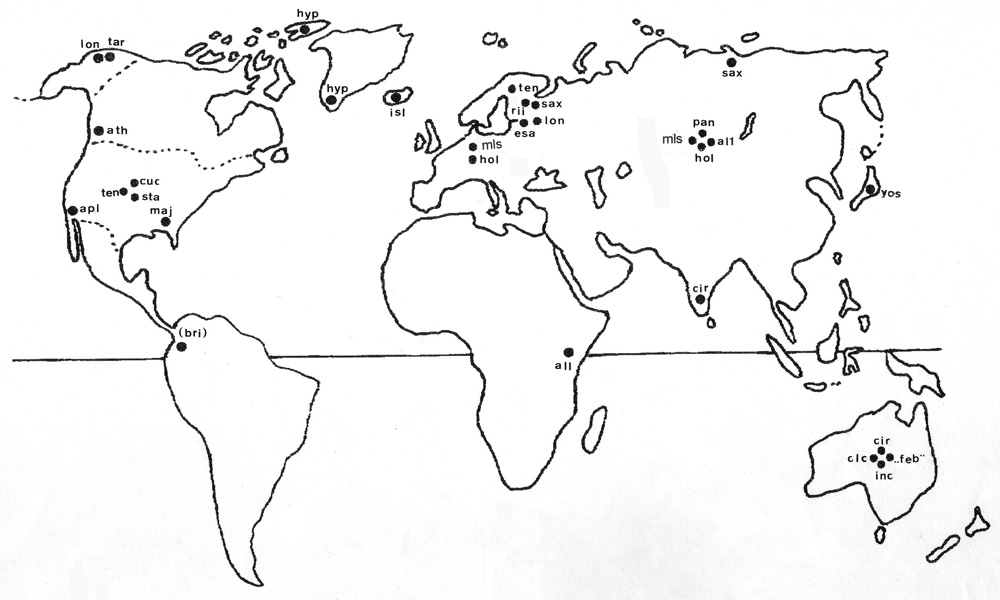 |
I saw myself that some of the "central" pattern named by Keyl are continent-overlapping and cytocomplex-overlapping and come together in some species in different continents (C. longistylus in Europe, C. alluaudi in Afrika and C.spec.Apple Valley in N-Amerika) which are a kind of "ancestor" species with "basic" patterns , at least in chromosome arms A,E and F. The "basic" pattern are sometimes present in many species ,i.e. are "common" (have a "central" position)
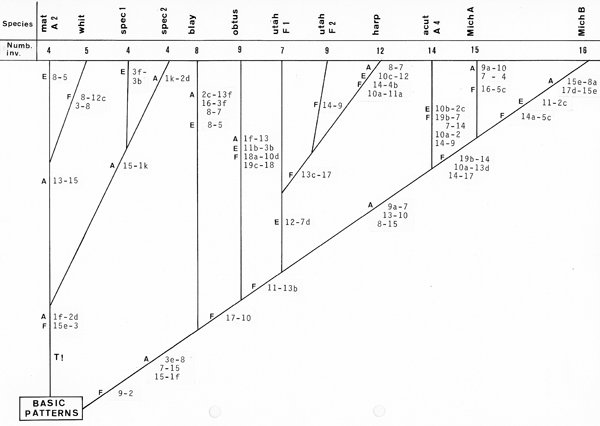
In an updated scheme, I can show the world-wide distribution of the pattern of C. holomelas in chromosome arm A (Fig. 7) 1-2c 10-12 3-2d 9-4 13-19. It is present in at least 27 species all over the world (bearing in mind, that chromosome investigations in Afrika and South America are very scarce). This picture may be explained by extreme migration activity of the species carrying this pattern or by independent incidence of the inversions leading to this pattern on different continents or alternatively (my opinion) as a "basic" pattern having existed before the separation of the main cytocomplexes (possibly out of the times of Gondwana-land, ca. 200 Mio years back) and then remaining on the different continents until recent times. It can well be that it was sometimes retained by selective forces as supposed recently by Guryev et al 2001 : it is indeed sometimes confined to typical relic biotopes like high mountains or swamps. But it can hardly be originated by these forces.
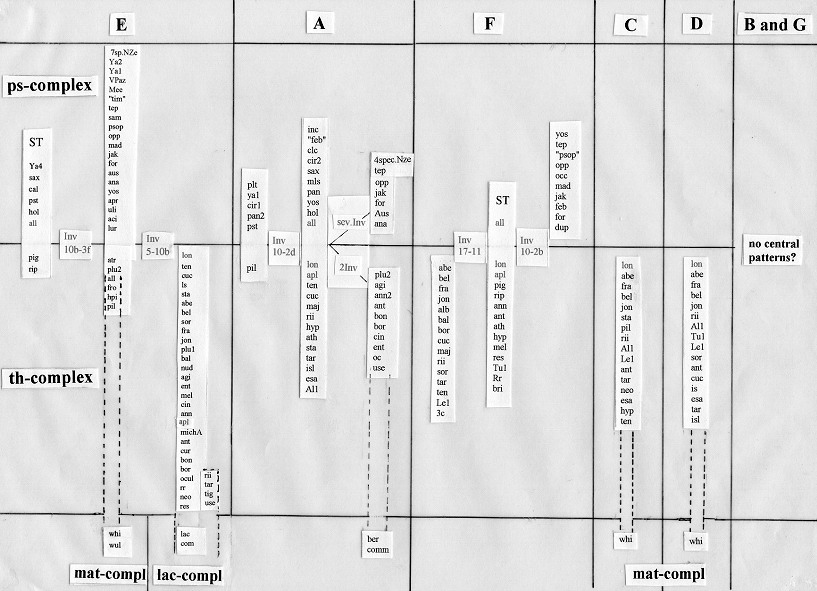 |
Also for "common" pattern I have an updated picture (Fig. 8). It shows also in arms A and F more than one "centres"(some of them complex-overlapping, others complex-specific) and includes arms C and D, where "centers" appear only in the thummi-complex. In arms B and G only few species have identical patterns. From the left to the right you may see a scale from more "conservative" to more "progressive" chromosome arms. Also note the occurrence of C. longistylus in the centers of 5 chromosome arms, we will come back to this statement in Fig.11.
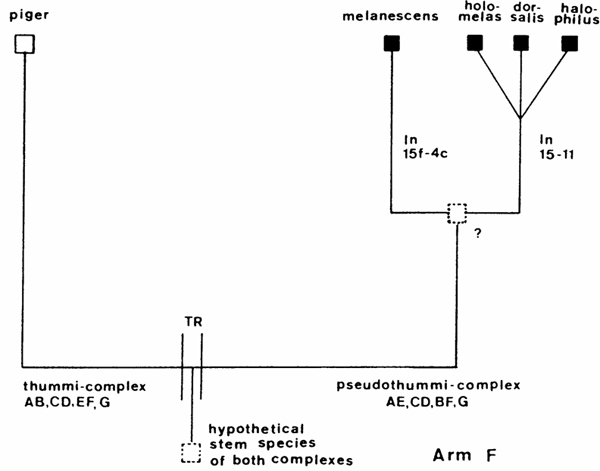 |
An other advantage of Wagner-nets is the possibility of predictions. Wuelker(1980) based on Keyl (1962) showed an example Fig.9): If two taxa of the pseudothummi-complex can be related by one inversion step to C. piger (thummi-complex), but differ by two inversion steps from each other than the banding pattern of C. piger should exist also in the pseudothummi-cytocomplex : C. alluaudi in Afrika was filling the gap.
2) Statistical treatment
The next step was statistical treatment. Assuming that species with the most frequent neighbourhood of bands (Wuelker et al.1984, 1989) or the minimum of breaks leading to other species (Shobanov 2000) may have a basic position, the phylogentic status of many Chironomus-species has been calculated with use of distance-matrices (Fig. 10). In the pseudothummi-and thummi-complexes the species show a comparable difference between "basic" and "derived" species, in the thummi-complex just those species are at the basis, which were declared earlier as "basic",(i.e. in central position, continent-overlapping and cytocomplex-overlapping). The species declared as "basic" in Shobanov 2000 (cucini, tardus, tenuistylus) are very close to our "ancestors" (longistylus, alluaudi, spec.Apple Valley)
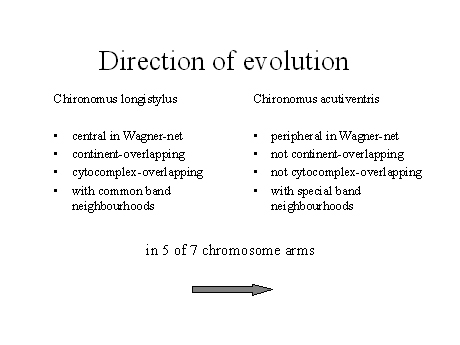 |
3) Direction of evolution
For comparison, I am showing the "basic" C. longiventis and the "derived" C. acutiventris (Fig.11). One of the arguments alone may not provide enough evidence (e.g.the rule "common is primitive" has of course no overall validity) but all four together make an evolutionary direction from left to right highly probable.
4) Phylogenetic trees
 |
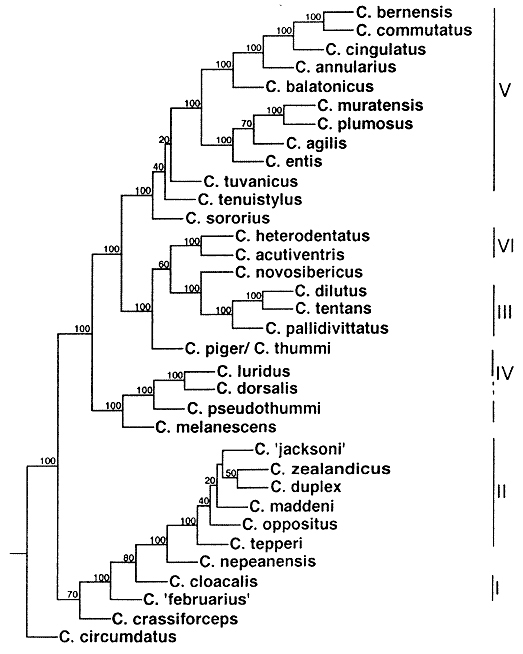
It is possible to construct phylogenetic trees on basis of distance matrices, simply beginning with "ancestors " and following step by step the inversions (Fig. 12). Nevertheless, distance analysis is not well recommended in phylogenetic systematics, in being "phenetic" i.e. not distinguishing trivial and informative characters. Other numerical methods like maximal parsimony or maximum likelihood may be more appropriate. J.Martin (in Guryev et al. 2001) recently published a cytological phylogenetic tree (Fig. 13) based on chromosome arms A, E and F and on parsimony (mixed of Wagner and Fitch-Sokal) including for the first time south-east asian species, but not including our "ancestor" species, nor banding pattern of chromosome arms C and D. I have the hope that after removing these deficiencies, a truely representative phylogenetic tree can be achieved.
 |
I have tried to show that chromosomes are playing an important role in Chironomid research, comparable to some extent to the better known Drosophilids or Culicids. We have to consider, however, that the advantage of giant chromosome analysis is limited to only few groups in the animal kingdom, and that phylogenetic considerations are only possible if bands can be homologized.
August Thienemann, who was a fan of the genus Chironomus and used it in his famous lake typology, would certainly have enjoyed modern progress in the knowledge of his beloved Chironomids.
Acton, A.B. 1959. A study of the differences between widely separated populations of Chironomus (=Tendipes) tentans (Diptera). Proc. Roy. Soc. B 151: 277-296.
Butler, M.G., I.I. Kiknadze, V.V. Golygina, J. Martin, A.G. Istomina, W.F. Wuelker, J.E. Sublette, and M.F. Sublette.1999. Cytogenetic differentiation between palaearctic and Nearctic populations of Chironomus plumosus L. (Diptera, Chironomidae). Genome 42: 797-815.
Dévai, Gy., M. Miscolczi, and W. Wülker. 1989. Standardization of chromosome arms B,C, and D in Chironomus (Diptera: Chironomidae).- Acta. Biol. Debrecina, Suppl. Oecol. Hungar. 2: 79-92
Guryev, V., I. Makarevitch, A. Blinov, and J. Martin. 2001. Phylogeny of the genus Chironomus (Diptera) inferred from DNA sequences of mitochondrial Cytochrome b and Cytochrome oxidase I. Mol. Phyl. Evol. 19:9-21.
Keyl, H.G. 1961. Chromosomenevolution bei Chironomus. I. Strukturabwandlungen an Speicheldruesen-Chromosomen. Chromosoma 12: 26-47.
Keyl, H.G. 1962. Chromosomenevolution bei Chironomus. II. Chromosomenumbauten und phylogenetische Beziehungen der Arten. Chromosoma 13: 464-514.
Keyl, H.G. 1966. Lokale DNA-Replikationen in Riesenchromosomen. In: Sitte, P. (edit.) Probleme der biologischen Reduplikation. 3.wiss.Konf.Ges.dt.Naturf.Aerzte, Wien 1965
Kiknadze, I.I., A.I. Shilova, I.E. Kerkis, N.A. Shobanov, N.I. Zelentsov, I.P. Grebenuk, A.G. Istomina, and W.A. Prasolow. 1991. Karyotypes and larval morphology in the tribe Chironominae. Atlas(in Russ.). Novosibirsk, Nauka Publishers 113.p.
Kiknadze.I.I., M.G. Butler, K.K. Aimanova, L.I. Gunderina, and J.K. Cooper. 1996. Geographic variation in the polytene chromosome banding pattern of Holarctic midge Chironomus (Camptochironomus) tentans (Fabricius).- Canadian Journal of Zoology 74, 171-191
Kiknadze, I.I., M.G. Butler, V.V. Golygina, J. Martin, W.F. Wuelker, J.E. Sublette, and M.F. Sublette. 2000. Intercontinental karyotypic differentiation of Chironomus entis Shobanov, a holarctic member of the plumosus group (Diptera, Chironomidae). Genome 43: 857-873.
Linevich, A.A. 1981. Chironomids from Baikal and surroundings (in Russian).- Novosibirsk: Idatelstvo Nauka, Sibirskoye Otdeleniye, 1-152.
Martin, J.1979. Chromosomes as tools in taxonomy and phylogeny of Chironomidae. Ent.Scand. Suppl.10:67-74.
Maksimova, F.I. 1976. The karyotype of Chironomus plumosus L. from the Ust’-IIzhora wild population of Leningrad region (in Russian).- Tsitologiya 18: 1264-1269
Proviz,V. B.R. Goddeeris, and V. Belkov. 1994. Speciation in Baikal Chironomidae (Diptera): An introduction.-Arch.Hydrobiol. Beih.Ergebn.Limnol.44:327-334.
Rempel, J.G. 1940. Intersexuality in Chironomidae induced by nematode parasitism. J.exp.Zool:84, 261-289.
Shobanov, N.A., I.I. Kiknadze, and M.G. Butler. 1999. Palaearctic and nearctic Chironomus (Camptochironomus) tentans Fabricius are different species. Ent.scand. 30: 311-322.
Shobanov, N.A. 2000. Phylogenetic problems of the genus Chironomus Meigen (Diptera, Chironomidae).- In Hoffrichter, O. edit.: Late 20th century research in Chironomidae: An anthology from the 13th International Symposium in Chironomidae, Shaker-Verlag, Aachen, p.231-244.
Thienemann, A. 1936. Alpine Chironomiden (Ergebnisse von Untersuchungen in der Gegend von Garmisch-Partenkirchen, Oberbayern).- Arch. Hydrobiol 30: 167-262.
Townes, H.K. 1945. The nearctic species of Tendipedini (Diptera) Tendipedidae (=Chironomidae.- Amer.Midl.Nat. 14: 206.
Velichko, A.A., Yu.M. Kononov, and M.A. Faustova. 1997. The last glaciation of Earth: Size and volume of ice-sheets.- Quaternary International 41/42: 43-51.
Wülker, W. 1961. Untersuchungen über die Intersexualität der Chironomiden (Dipt.) nach Paramermis-Infektion.- Arch.Hydrobiol. Suppl.25, 127-181.
Wülker, W. 1962. Parasitäre und nichtparasitäre geschlechtliche Aberrationen bei Chironomiden (Dipt.).- Zool.Anz., Suppl.25: 32-39.
Wülker, W. 1980. Basic pattern in the chromosome evolution of the genus Chironomus (Diptera).- Z. zool. Syst. Evol.-forsch. 18: 112-123.
Wülker, W., and A.M. Klötzli. 1973. Revision der Gattung Chironomus Meig. IV.Arten des lacunarius (commutatus-) Komplexes.- Arch. Hydrobiol.72: 474-489
Wülker, W., G. Lörincz, and Gy. Dévai. 1984. A new computerized method for deducing phylogenetic trees from chromosome inversion data.- Z.zool.Syst.Evol.forsch. 22: 86-91.
Wülker, W., Dévai, Gy., Dévai, I. 1989. Computer-assisted studies of chromosome evolution in the genus Chironomus (Dipt.) Comparative and integrated analysis of chromosome arms A,E and F. .-Suppl.Oecol.Hungar. 2: 373-387.
Wülker, W., J.E. Sublette, and J. Martin. 1991. Chironomus utahensis Malloch and Chironomus harpi new species and their karyosystematic relationship to other species of the decorus-group of Chironomus.- Spixiana 14:71-94.
abe = aberratus
aci = acidophilus
acu = acutiventris
agi = agilis
Al 1 = spec.Altai 1 (Kiknadze)
alb = albimaculatus
all = alluaudi
ana = analis
ann = annularius
ant = anthracinus
apl = spec.Apple
Valley (Wuelker)
apr = aprilinus
ath = athalassicus
atr = atrella
aus = australis
bal = balatonicus
bel = beljaninae
ber = bernensis
bly = blaylocki
bon = bonus
bor = borokiensis
bri = spec.Las Brisas (Wuelker)
cal = calipterus
cin = cingulatus
cir = circumdatus
clc = cloacalis
com = commutatus
cra = crassicaudatus
crm = crassimanus
cuc = cucini
cur = curabilis
dor = dorsalis
dup = duplex
ent = entis
esa = esai
feb = februarius
for = forsythi
fro = frommeri
har = harpi
het = heterodentatus
hol = holomelas
hpi = heteropilicornis
hyp = hyperboreus
inc = incertipennis
is = spec. Isabel Lake
(Kiknadze)
isl = islandicus
jak = jaksoni
jon = jonmartini
lac = lacunarius
Le1 = spec.Lena 1 (Kiknadze)
lon = longistylus
lur = luridus
mad = maddeni
maj = major
mat = maturus
Mee =
spec. Meerenberg (Wuelker)
mel = melanotus
Mich A und B = spec.
Lake Michigan (Wuelker)
mls = melanescens
mur = muratensis
neo = neocorax
nud = nuditarsis
obt = obtusidens
occ = occidentalis
ocul = oculatus
opp = oppositus
pan = pannkratovae
pig = piger
pil = pilicornis
plt = plumatisetigerus ( = circumdatus?)
plu = plumosus
psop = pseudoppositus
pst = pseudothummi
res = reservatus
rii = riihimakiensis
rio = spec.Rio de Janeiro (Wuelker),
rip = riparius
rr = spec.rr (Martin)
sam = samoensis
sax = saxatilis
sok = sokolovae
sor = sororius
sta = staegeri
sti = stigmaterus
ter = tardus
ten = tenuistylus
tep = tepperi
tim = timmsi
tig = tigris
tra = transvaalensis
Tu1 = spec. Tuva 1
(Kiknadze)
uli = uliginosus
use = usenicus
uta = utahensis
Vpaz = spec. Villa Paz (Wuelker)
whi = whitseli
WOC = spec. WOC
(Wuelker)
wul = wulkeri
Ya = spec. Yakutsk (Kiknadze)
yos = yoshimatsui
converted to html by Ethan Bright, 25 November 2007; last updated: 01-Dec-2007