 We commonly rear midges from field collected egg masses or from egg masses laid in the laboratory.nbsp; nbsp; There have been a number of rearing techniques published, but we have found the following to be the most useful:
We commonly rear midges from field collected egg masses or from egg masses laid in the laboratory.nbsp; nbsp; There have been a number of rearing techniques published, but we have found the following to be the most useful: Rearing medium
 We commonly rear midges from field collected egg masses or from egg masses laid in the laboratory.nbsp; nbsp; There have been a number of rearing techniques published, but we have found the following to be the most useful:
We commonly rear midges from field collected egg masses or from egg masses laid in the laboratory.nbsp; nbsp; There have been a number of rearing techniques published, but we have found the following to be the most useful:
Rearing medium
0.04% (w/v) NaCl - this was recommended for Chironomus tentans by Case & Daneholt (1978). While good for large species, smaller species may require additional nutrients.
Walter's solution - developed for European species which normally live in Na+- CO3- - waters, by Walter (1973): To 100L water is added: 0.5g NaHCO3, 3.5g NaCl, 2.7g CaCl2, 0.2g KH2PO4, 3.0g MgSO4, 1mL 1% FeCl3.
Martin's solution - developed for Australian species that live in Na+- Cl- - waters, by Martin (Martin et al. 1980): To 4L of water add 1mL/L of the following salt concentrates (each in 100mL water) - 1.0g NaHCO3; 5.0g NaCl; 1.0g CaCl2; 0.2g KH2PO4; 1.0g MgSO4; 1.8g MgCl2. To this is added 0.2mL/L of a 0.1% FeCl3 solution (to help ensure sufficient Fe for the multiple globins); 0.25mL/L of solutions of 0.6g/100mL Thiamine HCl (this is not needed if the food used has vitamin B added, as many fish foods do. Biever (1971) for example found that addition of vitamins to Dog Kisses, which already contained 0.13 mg thiamine mononitrate, actually lengthened developmental time and reduced the survival rate)) and a mixture of 5g NH4C2H3O2 (since many Chironomus species do well in water polluted by ammonium containing fertilizers), 0.1g MnCl2, and 0.1g KI in 100mL water (these latter to provide minor elements).
Three to five shredded Kleenwipe, or 8 to 10 shredded single-ply toilet tissues (avoid recycled paper products, as these appear to be detrimental to the larvae) are added for the larvae to use as additional food and from which to build their tubes. If wear on the larval mentum is not of concern, the addition of a thin layer of stone dust (i.e. the dust produced when stones are cut with a power saw - usually available from building suppliers or plant nurseries) will produce larger, healthier larvae with better quality polytene chromosomes. Additional tissues may be added each week as needed.
Moderate aeration is required to provide sufficient oxygen for the larvae. An aquarium stone will build up algal and other deposits and may be a source of contamination between containers, so I use about 14 or 16 gauge hypodermic needles, which can be easily cleaned. Some people believe aeration should not be supplied in the first few days after setting up an egg mass, but we have found no problem with providing aeration right from the start in order to prevent the formation of a film of scum on the water surface.
Temperate zone species are reared at 20oC, tropical species at 25oC, with a light regime including a morning and evening 'twilight' period: 1 hr low light; 14hrs full light; 1hr low light; 8hrs dark. The actual wattage required will depend upon the size of the room being lit. Unfortunately the introduction of 'low wattage' globes is making it difficult to get the 25W or lower incandescent globes that are best for the 'twilight' periods.
Feeding
A variety of foods have been recommended for rearing chironomids, including powdered nettle leaves (Case & Daneholt 1978), TetraMin fish food, powdered milk, Trout Starter 1c (ie. the finest food given to fingerling trout) which is ground with a mortar and pestle, and even chocolate coated dog kisses (Biever 1965).
We use a mixture of fish foods: usually a floating pellet and a Chiclid pellet, ground and fed at the rate of 0.2g (for a single egg mass) or 0.4g (for a mass rearing cage) per week. Availability of particular brands varfies with time and place, but any sinking pellet or stick fish food is suitable after grinding.
I have found that feeding preferences of larvae may vary between continents, for example Australian Chironomus species do not seem to do well on the crushed nettle leaves so favoured for European species.
 This technique has been used for rearing, but not necessarily breeding,
species of Chironomus, Kiefferulus, Dicrotendipes, Einfeldia, Conochironomus, Microtendipes, Polypedilum, Riethia, Tanytarsus, Paratanytarsus,
Cricotopus, Procladius and Psectrocladius.
This technique has been used for rearing, but not necessarily breeding,
species of Chironomus, Kiefferulus, Dicrotendipes, Einfeldia, Conochironomus, Microtendipes, Polypedilum, Riethia, Tanytarsus, Paratanytarsus,
Cricotopus, Procladius and Psectrocladius.
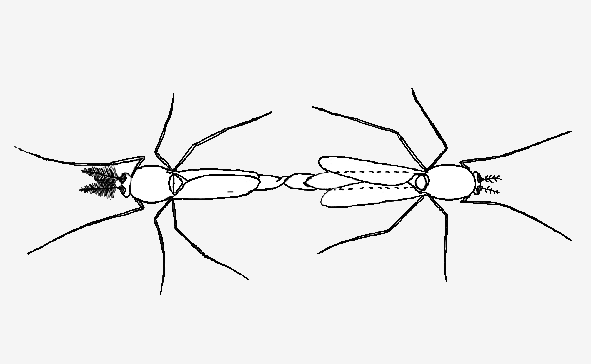
Some species will breed in the rearing cage, but for others the adults need to be collected and put into a separate breeding cage about 1m below the room lights. We have found that any Australian species that is going to breed in the laboratory will breed in a 30cm cube cage, comprising a wooden frame covered with mesh (Top row in figure). A white enamel dish of water is placed on the bottom of the cage, into which the females will deposit their eggs.
In some cases only about 8 - 10 males are necessary, but for others at least 20 - 30 males are needed before successful mating will occur. The ease of mating under laboratory conditions can vary markedly between closely related species. Species with the enlarged hypopygium that commonly accompanies mating on the substrate, are usually the easiest to breed under laboratory conditions.