The Unusual Sex Determination System ofChironomus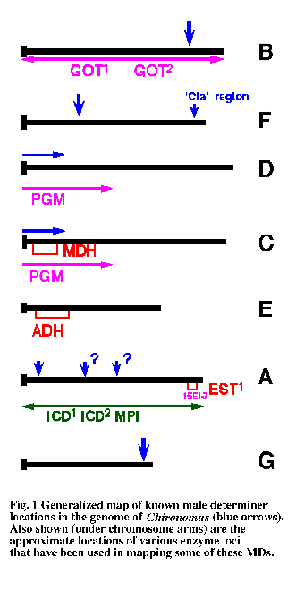
Chironomids generally have no cytologically recognized sex chromosomes, the polytene chromosomecomplement of males and females appearing identical. However in some cases there are inversion or translocation sequences which occur only in males, and serve to mark the Y chromosome (Beermann 1955; Acton 1957; Martin 1962; Newman 1977). In order to recognize this it is necessary to sex the larvae.
The most differentiated case of sex chromosomes has been reported in Telmatogeton hirtus, where there is an XY1Y2system, in which part of the Y chromosomes has become heterochromatic(Newman 1977).
The Y chromosome can also be identified in other cases by sex linkage of enzyme loci (Martin & Lee 1988b), or even by experimental induction of chromosomal rearrangements of the Y chromosome (Martin 1974).
These data clearly indicate that sex determination is a male dominantsystem. There have been reports of female dominant sex determination in the genus Polypedilum (Martin 1966; Porter& Martin 1977) and also in certain North American populations ofChironomus dilutus (Thompson 1971: Thompson & Bowen 1972). While this seems to be correct for Polypedilum,the situation in C. dilutus appears to result from a misinterpretation of the data (Martin & Lee 1984).
It is difficult to accurately map the chromosomal locations ofthese MD genes, but it appears that there may be a relatively small numberof sites (Fig. 1). The most common sites differ in different geographic regions, e.g. on arm F in Europe but near the CD centromere in Australia (Martin 2010).Often the MD site changes at speciation, and there is a possibility that MDsite may be polymorphic in some species, although it may also be that thesedifferent MD sites actually indicate the presence of cryptic species(Martin & Lee 1988a).
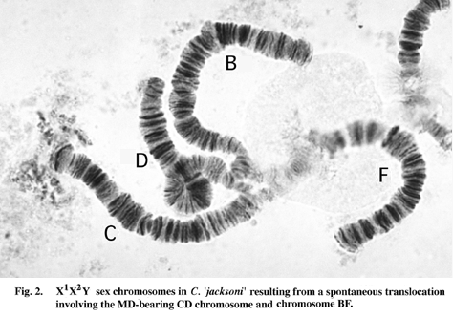
A spontaneous mutation in a laboratory strain of C. 'jacksoni' led to the creation of a new sex chromosome - but not a change in MD site. The MD is near the centromere of the CD chromosome, but a whole arm translocation has converted the arm combination from BF, CD to BD and CF. The normal CD chromosome, rather than either translocated chromosome, carries the MD locus, so the translocation effectively creates an X1X2Y sex chromosome system (Fig. 2). In balanced gametes both the CF and BD chromosomes segregate together and may be passed to either sex.

It seems likely that the sex determination pathway will berelated to that of Drosophila melanogaster subsequent to the actionof the sexlethal gene, which does not appear to be involved in sexdetermination in other groups of Diptera (Martin & Lee 2000). Data from other insects indicate that it will be centred on the doublesex gene (Ohbayashi et al. 2001; Hediger et al. 2004; Cho et al. 2007). Cho et al. (2007) proposed that, where female is the default sex, the female-specific splicing of doublesex is the default situation, with transformer suppressing splicers of doublex. In males, transformer is non-functional, so that the splicers mediate the splicing of the male-specific variant of doublesex. Hediger et al. (2010) have extended this model to show continuous activity of transformer (Mdtra)is required to maintain female development and that male development is established by regulatory genes (the autosomal male determining genes) that regulate transformer, to prevent establishment of the auto-feedback loop required for continuous activity. This fits quite well with the model of sex determination in Chironomus proposed by Martin & Lee (2000) which proposed that male development could be mediated by genes which down regulate the tra genes or alter the transcription of the doublesex gene to switch the pathway from the default female development to that of male development (Fig. 3). These could be genes that act at different points in the sexual development pathway. The original scheme of Martin & Lee (2000) may need to be modified by limiting of the function of the various MD genes to that of regulating the early activity of tra to prevent establishment of the auto-feedback loop, or mutations of tra itself.
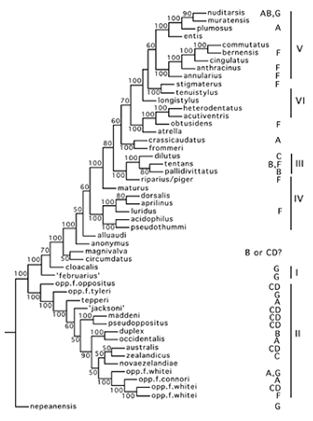
Consensus mixed parsimony cytological tree, rooted on C. nepeanensis, with MD location indicated on the right. (from Martin, 2010).Another possibility is that the MD gene is associated with a transposable element (TE), which can insert into particular sites on the chromosome where recognition sequences for the TE occur (Martin & Lee 1988a). Such a gene could be a suppressor of tra.
Since Mdtra was originally identified as a female sex determiner, it is interesting to speculate that the dominant female determiner on arm G of Polypedilum nubifer (Martin 1966; Porter & Martin 1977) is the tra gene, and that the MD on arm G in Chironomus is an effectively non-functional tra gene.
This suggestion is supported by a phylogenetic analysis of MD location. This indicates a marked difference in MD location of southern hemisphere species (arm C) and the northern hemisphere species (arm F), but of most relevance to the location of tra is that the arm G site is found in the most basal species (Martin 2010, see Figure below). The presence of a sex determining gene that is basal on the equivalent chromosome to the female determining gene of P. nubifer is strongly suggestive that this could be the site of tra.We have been interested in cloning and identifying the MD gene that occurs on arm G (Fig. 1). In order to achieve this we are accumulating cloned genes that have been mapped to the distal end of the arm. Part of the strategy is to sequence this region of the chromosome, to determine the relative positions of these genes and to locate the MD gene in relation to them.
Another approach is to obtain clones of known sex determination genes from Chironomus, to see where these map relative to known MD sites. So far only double sex is known to have been cloned, and it is stated that this is not at any known MD locus (E.R. Schmidt, pers. comm.).
References
Acton, A.B. 1957. Sex Chromosome inversions in Chironomus. Amer. Nat. 91:57-59.
Beermann, W. 1955. Geschlechtsbestimmung und Evolution der genetischen Y-Chromosomen bei Chironomus. Biol. ZentralBl. 74: 525-544.
Cho, S., Huang, Z.Y. and Zhang, J. 2007. Sex-specific splicing of the honeybee doublesex gene reveals 300 million years of evolution at the bottom of the insect sex-determination pathway. Genetics 177: 1733-1741.
Hediger, M., Burghardt, G., Siegenthaler, C., Buser, N., Hilfiker-Kleiner, D., et al. 2004. Sex determination in Drosophila melanogaster and Musca domestica converges at the level of the terminal regulator doublesex. Dev. Genes Evol. 214: 29-42.
Hediger, M., Henggeler, C., Meier, N., Perez, R., Saccone, G. and Bopp, D. 2010. Molecular characterization of the key switch F provides a basis for understanding the rapid divergence of the sex-determining pathway in the housefly. Genetics 184, 155-170.
Martin, J. 1966. Female heterogamety in Polypedilum nubifer(Diptera: Nematocera). Amer. Nat. 100: 157-159.
Martin, J. 1962. Interrelation of inversion systems in the midgeChironomus intertinctus (Diptera: Nematocera) I. A sex-linked inversion. Aust. J. Biol. Sci. 11: 666-673.
Martin, J. 2010. A phylogenetic analysis of the change in genomic location of the sex determining region in the genus Chironomus (Diptera: Chironomidae). VOGiS Herald 14: 62-69.
Martin, J and Lee, B.T.O. 1984. Are there female heterogametic strains ofChironomus tentans Fabricius? Canad. J. Genet. Cytol.26: 743-747.
Martin, J. and Lee, B.T.O. 1988a. Sex determiners and speciation in the genus Chironomus. Pacific Sci. 42: 51-55.
Martin,J., and Lee, B.T.O. 1988b. The chromosomal location of the malate dehydrogenase and the phosphoglucomutase loci in Chironomus andtheir relationship with a sex determining region. Genetics(Life Sci. Adv.) 7: 57-63.
Martin, J. and Lee B.T.O. 2000. Sex determination in Chironomus and the Drosophila paradigm. In: "Late 20th Century Research on Chironomidae: an Anthology from the 13th International Symposium on Chironomidae." (Ed. O. Hoffrichter), Shaker Verlag, Aachen pp. 177-181.
Newman, L.C. 1977. Chromosomal evolution of the HawaiianTelmatogeton (Chironomidae, Diptera). Chromosoma 64: 349-369.
Ohbayashi, F.M., Suzuki, M.G., Mita, K., Okano, K. and Shimada, T. 2001. A homologue of the Drosophila doublesex gene is transcribed into sex-specific mRNA isoforms in the silkmoth, Bombyx mori. Comp. Biochem. Physiol. B Biocem. Mol. Biol. 128: 145-158.
Porter, D.L. and Martin, J. 1977. The cytology of Polypedilumnubifer (Diptera: Chironomidae). Caryologia30: 41-62.
Thompson, P.E. 1971. Male and female heterogamety in populations ofChironomus tentans (Diptera: Chironomidae). Canad. Entomol. 103:369-372.
Thompson, P.E. and Bowen, J. 1972. Interactions of differentiatedprimary sex factors in Chironomus tentans. Genetics 70: 491-493.
[ LAB RESEARCH| LAB PERSONNEL| MARTIN LAB HOME ] |